Abstract
Introduction:
Severe aplastic anemia (SAA) is a rare hematopoietic stem cell disorder characterized by hypocellular marrow and pancytopenia. Multiple factors play an important role in treatment approach include age, comorbidities, degree of pancytopenia and availability of stem cell donor to either immunosuppression irrespective (IST) or allogenic hematopoietic stem cell transplant (alloSCT). The use of nonmyeloablative conditioning regimen has improved the outcomes, however the choice for post-transplant GVHD prophylaxis remain a topic of debate. The use of mycophenolate mofetil (MMF) has been used as an alternative for methotrexate (MTX) as has shown to be associated with lower incidence of mucositis and shorter time to engraftment.
Methods:
We retrospectively evaluated consecutive adult patients with SAA who underwent alloSCT at Karmanoc Cancer Institute. All patients received fludarabine, cyclophosphamide and antithymocyte globulin for conditioning regimen with calcineurin inhibitors (CNI) and MMF for GVHD prophylaxis. MMF was started at day -3 at 15 mg/kg three times daily and stopped at day +30 in the absence of active GVHD. The primary objectives were to estimate cumulative incidence of acute (aGVHD) and chronic GVHD (cGVHD) and overall survival (OS). Secondary objectives were to evaluate time to engraftment, days of hospitalization and incidence of mucositis.
Results:
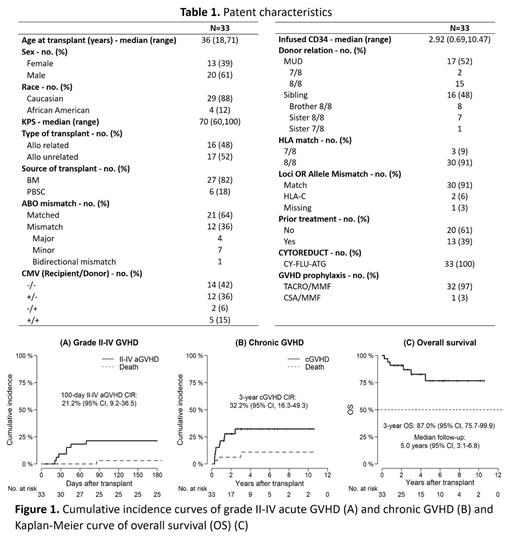
From January 2005 and May 2019, 33 patients with SAA underwent alloSCT. Patient characteristics are detailed in Table 1. Median age was 36 years (range, 18-71). Twenty-seven patients received bone marrow stem cells (82%) and six patients received peripheral blood stem cells (18%). Thirty patients (91%) received 8/8 HLA matched donor and three patients (9%) received 7/8 HLA matched donor. Sixteen patients (48%) received stem cells from sibling donor and 17 patients (52%) received stem cells from unrelated donor. Thirteen patients (39%) had received IST prior to alloSCT, and 20 patients (61%) received upfront alloSCT. For GVHD prophylaxis all patients received MMF and CNI (tacrolimus=32, and cyclosporine=1). Median time from diagnosis to transplant was 15.8 months for patients who received IST prior to alloSCT and 2 months for patients who received upfront alloSCT. Median time to platelet engraftment was 13.5 days and neutrophil engraftment was 12 days, while one patient experienced graft failure. The median number of days for hospital stay were 25 days. Four patients (11%) developed grade I-II mucositis, no grade III-IV mucositis was observed in the first 30 days and 6 patients had CMV reactivation. The 100-day cumulative incidence rate of grade II-IV aGVHD was 21.2% (95% CI, 9.2 - 36.5), grade III-IV aGVHD was 9.1% (2.3-21.9) and 1-year CIR of cGVHD was 21.2% (95% CI, 9.2-36.5). Comparing patients who received IST prior to alloSCT versus upfront alloSCT, the 100-day CIR of grade II-IV aGVHD was 30.8% (95% CI, 8.2 - 56.5) and 15% (95% CI, 3.6 - 34.0), respectively, (Gray p=0.26) and the 3-year CIR of cGVHD was 39.6% (95% CI, 13.1 - 65.5) and 27.8% (95% CI, 9.2 - 50.3), respectively, (Gray p=0.37). Comparing patients who received alloSCT from related versus unrelated donor, 100-day CIR of II-IV aGVHD was 12.5% (95% CI, 1.9 - 33.6) and 29.4% (95% CI, 10.2 - 51.9), respectively, (Gray p=0.26), and the 3-year CIR of cGVHD was 34.2% (95% CI, 11.4 - 58.9) and 29.4% (95% CI, 10.1 - 52.0), respectively (Gray p=0.90). Median follow up of surviving patient was 5 years (95% CI, 3.1-6.8). Three-year OS was 87% (95% CI, 75.7- 99.9) and median OS was not reached. Six patients died by the time of the analysis, one patient died from graft failure (86 days after transplant from 8/8 HLA MUD), two patients died due infectious complications (808 days and 1637 days after transplant), three patients died due to multiorgan failure (215, 297 and 1097 days after transplant).
Conclusion:
Our data with use of CNI and MMF for GVHD prophylaxis for SAA following alloSCT with nonmyeloablative conditioning regimen showed that the rate of mucositis was low, engraftment time was rapid, and hospitalization was short, while OS, rates of acute and chronic GVDH were comparable to previously published rates with CNI and MTX-based GVHD prophylaxis.
Modi: Genentech: Research Funding; Seagen: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Membership on an entity's Board of Directors or advisory committees. Deol: Kite, a Gilead Company: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal